FSU chemist awarded $3.4M grant to investigate protein complexes in bacteria that cause tuberculosis

A Florida State University chemistry researcher will use new funding to explore ways to halt tuberculosis, one of the world’s deadliest and most infectious diseases.
Professor of Chemistry and Biochemistry Yan-Yan Hu will use the five-year, $3.4 million National Institutes of Health grant to help identify resistance-breaking tuberculosis treatments by investigating the structures and interactions of key protein complexes in the mycobacteria that cause tuberculosis and how these interactions influence cell function.
“We plan to examine protein-protein interactions in functional complexes that are crucial to cell division,” Hu said. “The findings will help guide treatment targeting these crucial interactions, thus disrupting the cell division process. Our results will provide insights for identifying new strategies for combating drug resistance.”
Mycobacterium tuberculosis, Mtb, infects a quarter of the world’s population and is the world’s leading infectious disease killer, causing 1.25 million fatalities in 2024, according to the World Health Organization. Mtb often grows resistant to treatment because treatment for active tuberculosis requires a combination of antibiotics administered at specific intervals over six months to kill the bacteria. When patients fail to complete the full course of treatment, the bacteria adapt to survive the treatment cocktail in the future.
“It’s critical to understand this cell division because tuberculosis is becoming harder to treat as the mycobacteria continue to mutate and become more drug-resistant,” Hu said. “Many people also carry what we call dormant TB, or latent TB, which is when you carry the mycobacteria, but it doesn’t cause symptoms or spread to others until your immune system is compromised due to aging or other diseases. Between 5% and 10% of people with latent TB develop active TB in their lifetime.”
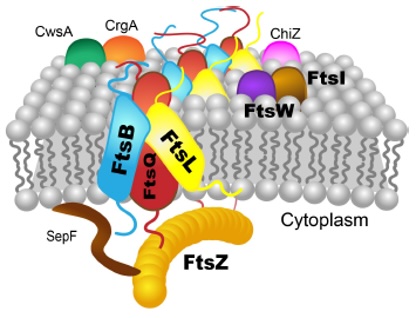
Hu and her group, supported by staff scientists and graduate students, will use the $2.2 million portion of the grant allotted to FSU to analyze a key set of protein complexes that drive cell division in Mtb: the QBL complex and the QZ interaction. The QBL complex connects important proteins in a cell’s cytoplasm to proteins in another part of the cell called the periplasm. The QZ interaction anchors the Z-ring, a crucial cytoskeletal structure in bacterial cell division, to the cell’s inner membrane.
Membrane proteins like these have a portion of their structure on each side of a membrane, such as the cell exterior and cellular interior, and they function to transmit nutrients into the cell or nucleus and transmit waste outside of the cell. They can also be used to communicate signals from the outside of the cell to the interior.
“Consequently, they’re very important proteins for cellular function; drugs that bind to such proteins and inhibit their protein function can disrupt the activities of the cell, such as cell division,” said Tim Cross, one of the study’s principal investigators and FSU professor of chemistry and biochemistry emeritus.
By altering interactions and structures within the complexes, the researchers will observe how these changes affect the cell division process and what alterations can make the process self-terminating.
Hu’s larger body of research is focused on developing and applying novel solid-state nuclear magnetic resonance, or NMR, techniques to study various systems ranging from biological systems like proteins to functional materials used in rechargeable batteries and fuel cells. NMR spectroscopy uses magnetic fields and radio-frequency pulses to analyze the structures and interactions of molecules at the atomic level, providing insights into both static structures and dynamic processes of the bacteria’s proteins.
“Yan-Yan is a very unusual researcher. She is a highly regarded battery chemist now branching into another field, which is fascinating and courageous,” Cross said. “Sometimes it takes a scholar with experiences outside of a particular field to bring in new insights, techniques and ideas that combine for unique breakthroughs in the research.”
The research, which involves biomolecular NMR techniques, computational biophysics, and cellular biology, is also supported by scientists at Harvard University, the University of Illinois Chicago, the University of Texas at Arlington, and the Nuclear Magnetic Resonance and Magnetic Resonance Imaging Facility at the FSU-headquartered National High Magnetic Field Laboratory.
To learn more about research conducted in the Department of Chemistry and Biochemistry, visit chem.fsu.edu. For more information about research conducted at the National High Magnetic Field Laboratory, visit nationalmaglab.org.
The post FSU chemist awarded $3.4M grant to investigate protein complexes in bacteria that cause tuberculosis appeared first on Florida State University News.